Proper preparation prior to surgery will improve animal recovery and surgical success, maximising the results you get from your telemetry system. See the sections below for suggestions regarding surgical tools, anesthesia and pain relief, and preparation of the animal before surgery. All surgery should be only be done with proper ethical approvals in place and in consultation with your institution’s veterinarian.
Surgical tools
Below is a list of general consumables and instruments that are useful during surgery. Unless otherwise stated, they should be packaged together and sterilized by autoclaving prior to surgery. Please see the application specific surgical videos for recommendations on additional surgical instruments or consumables specific to that application.
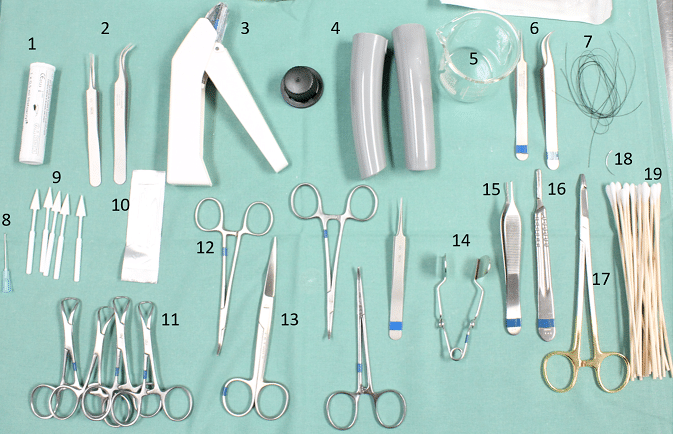
- Tissue adhesive e.g. Histoacryl or Vetbond (do not autoclave)
- Padded forceps for handling pressure catheter
- Skin stapler (do not autoclave) or wound clips
- Microscope handle and knob covers
- Beaker
- Fine forceps (straight and curved)
- Suture material e.g. 2-0 or 4-0 silk
- Catheter introducer made from bent 23G needle (BP surgery)
- Absorbent surgical spears (do not autoclave)
- Scalpel blade
- Towel clips for holding surgical drapes in place
- Haemostat clamps (2 or 3)
- Scissors
- Retractors
- Adson forceps
- Scalpel blade handle
- Needle holders
- Half circle needle
- Swab sticks
Not pictured
- Surgical drapes
- Gauze swabs 10cm x 10cm
- Sterile saline
Pre-operative care
Rats do not vomit so there is no need to withhold food or water before surgery.
Both analgesics and antibiotics work more effectively if given prophylactically before the start of surgery. Advice of your institution’s veterinarian should be sought with regard to specific drugs (and doses) but examples include the antibiotic Baytril (enrofloxacin) and analgesics Temgesic (buprenorphine) and/or Rimadyl (carprofen). We find that a subcutaneous or intramuscular injections at the time of induction of anesthesia the most convenient method of administration.
All animals should be kept warm during anesthesia, surgery and recovery. The use of a heating pad that has been turned on and warmed in preparation for the start of surgery will help maintain the rat’s body temperature and aid recovery.
Anesthetic induction and maintenance
Providing an adequate level of anesthesia is one of the critical aspects to chronic implantation of monitoring devices. The wrong anaesthetic will slow recovery or worse the animal will die during surgery.
The level of anesthesia should be assessed prior to and throughout surgery by monitoring the withdrawal reflex in response to a pinch of the paw pad throughout the surgery. A pulse oximeter sensor placed on the paw of the animal to measure heart rate and oxygen saturation can also be useful.
Recommended anaesthesia for rats and mice: Isoflurane provides excellent anaesthesia for surgery and recovery of animals.
Surgeries less than 45 min: Ketamine + xylazine may be useful but careful dosing and monitoring is required.
Isoflurane
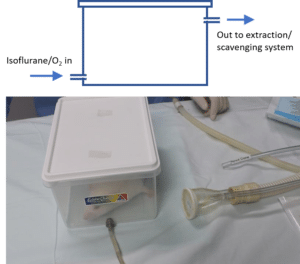
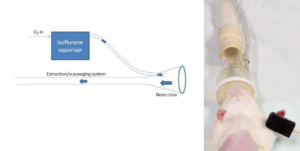
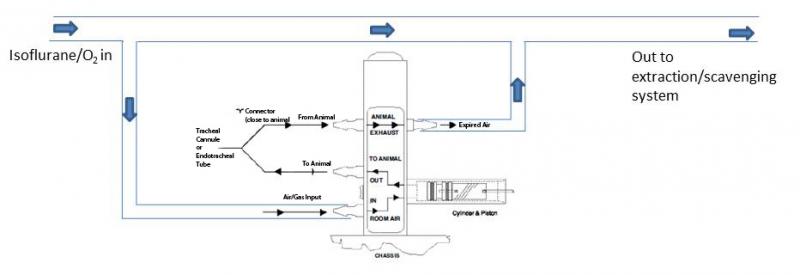
Isoflurane and other gaseous anaesthetics provide an easy and effective way of inducing and maintaining anesthesia. To induce anesthesia the animal can be placed inside an induction chamber or box. This box can be as simple as a plastic container with an inlet for the anaesthetic gas and an outlet to the exhaust. Once induced, anesthesia can be maintained using a nose cone for surgeries that do not require opening the thoracic cavity (intubation and ventilation is needed for these surgeries). An extraction or scavenging system must be used with gaseous anaesthetics.
Induction chamber
Nose Cone
Ventilator (required for LVP surgery)
Post-op pain relief
Animals that are in pain will be slower to recover from surgery. Animals should be observed carefully during the post-op period for signs of pain. In rats, this may be movement such as belly press, back arch, staggering etc. They may also seem reluctant to move and may appear ungroomed. Heart rate and blood pressure may also be elevated. Animals in pain will also tend to drink less so monitoring of fluid intake is a useful indicator. If these signs are present then further doses of pain relief should be considered.
Analgesics work best if given before surgery begins and are often continued for 1 or 2 days after surgery. It is important to discuss the type of surgery and the appropriate analgesia needs with your local institutional veterinarian. Examples of analgesia that might be used are Temgesic (buprenorphine, 30 µg/kg s.c.) for surgery involving a major body cavity or Rimadyl (carprofen, 4 mg/kg s.c.) for more minor surgery. Combining two different types of pain relief can maximise analgesic coverage and improve recovery. Mixing of analgesia such as Cartrofen with a treat food such as jelly or Nutella can be a good way to give additional analgesia during surgical recovery.
All users should consult their institution's veterinarian regarding specific drugs and doses.
Kaha Sciences is an ADInstruments brand. For all product information or support, please contact us.