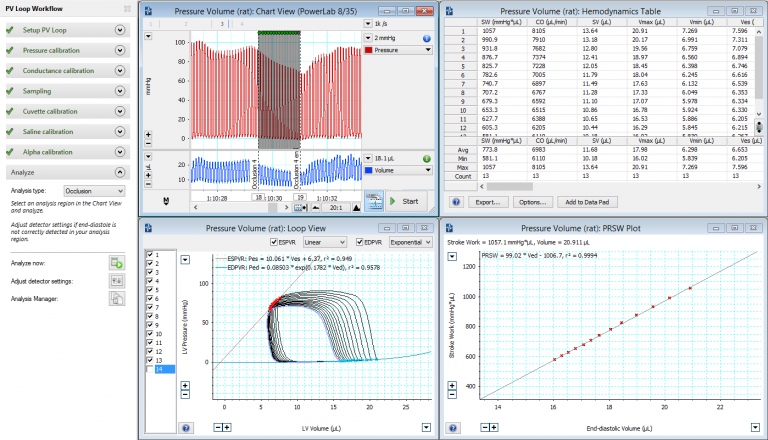
Left Ventricular Pressure-Volume via Carotid in Mouse
Instructions for carotid artery catheterization using a Millar 1F catheter
for left ventricular pressure-volume measurement in mice.
Surgery performed at the René Remie Surgical Skills Centre by Prof. René Remie. Animal handling and surgical procedures all strictly follow Dutch Legislation on Laboratory Animals and was approved by the IACUC.
Hardware:
- ADInstruments PowerLab 16/35 for data acquisition
- Animal BioAmp for ECG measurement
- Millar MPVS Ultra for Pressure and Volume measurement
Catheter:
- The Millar PVR-1035 PV catheter was connected to the MPVS Ultra.
Software used to record and analyze the pressure signal:
- ADInstruments LabChart
- LabChart's PV Loop Module was used to record and analyze the pressure-volume signal.
Anesthetic:
The mouse was anesthetized using injectable anesthetics and analgesics.
Procedure:
Make a simple catheter introducer
Before you begin the surgery, you can follow our step by step video instructions for making a simple catheter introducer. For a mouse, you need to use a 25 gauge needle, or smaller.
- With the bevel of the needle facing upwards, bend the tip almost 90degrees using a needle holder.
- You can also bend the needle a little to one side to prevent your fingers from being in the field of view when performing the catheterization.
- Attach the needle onto a syringe to create a simple catheter introducer.
Procedure begins: Incision
To start, make an incision from the level of the eyes to just above the sternum.
- Holding the tissue in the rostral direction, first make a small incision. Then, using the tissue holder and scissors, extend the incision along the neck.

Tip: Do not use scalpel blades as they can cause an enormous amount of blood loss.
This can be detrimental to the animal, especially a mouse.
- Cut through the connective tissue layer and with blunt dissection maneuver between the submandibular glands.
- Make sure that it is open enough in the caudal direction, so as to ensure the maximum available length of the carotid artery for catheterization.
Find the carotid artery
Once the tissue has been cleared away, you will see three muscles: namely, the sternohyoid, sternomastoid, and lower down, the digastric. These three muscles form a triangle. Inside this triangle, you will find the carotid artery.
- Using sharp forceps the sternohyoid muscle can be freed from the sternomastoid muscle.
- First place a hook to move the sternomastoid muscle out of the way.
- Use a second hook to move the submandibular gland out of the way.
- Using another hook on the other side, the sternohyoid muscle can be moved out of the way, so as to reveal as much of the carotid as possible.
- Separate the carotid artery from the surrounding tissue.
- Reposition the hook so that it includes the recently separated tissue to give maximum access to the artery.
- Similarly, free as much tissue as possible on the rostral side of the artery.
- You will notice the hypoglossal nerve, which indicates that you are near the carotid artery bifurcation.
- The bifurcation of the external and internal carotid artery can be clearly seen [video at 06.12]. You need to catheterize caudally from this.
Separate the vagus nerve from the carotid artery
- Very carefully open the perivascular sheath.
- Using blunt dissection make sure the vessel is separated from the nerve.
- Make sure the vessel and nerve are separated with enough length to allow for the tying off of the vessel.
Ligature
First, place the obstructive ligature.
- Make sure that the nerve is free from the vessel. Otherwise, there is the risk that you will tie the nerve together with the vessel in the ligature, which can cause erratic heart rates.
- Zoom out one step of the microscope to facilitate tying the knot.
- Move the ligature as far rostrally as possible.

Tip: By using the forceps and lifting them a little, one can add half a millimeter, which could make all the difference when inserting a PV catheter.
- Complete the knot.
- Add a drop of saline to the vessel to prevent it from drying out. It is interesting to note that a mouse can have 40 to 80 microlitres of insensible fluid loss per hour due to exposed tissue. For this reason, it is also advisable to counter this with a saline line in the left jugular vein for long term recordings.
- Next, place tension on the obstructive ligature by using some artery forceps.
Place the second ligature.
- Place a second ligature, which will be used to tie off around the catheter.
- Only tie half a knot and place the strands so that they are easily grasped when the catheter has been inserted.
- A third (safety) ligature will be placed later in the procedure.
Next, place the clamp.
- For this, a narrow jawed venous clamp will be used, also known as a Biemer clamp. Its closing force is high enough to occlude the artery in a mouse.
Insert the catheter

Tip: Before inserting the catheter, it’s always advisable to place the catheter on top of the vessel to check if the vessel length is enough to cover all electrodes in the catheter.
- To insert the catheter, puncture a hole using the catheter introducer, as close as possible to the obstructive ligature.
- Place the catheter under the needle and advance it into the artery as far as it will go. This will allow you to tie off the ligature behind the last electrode. Don’t over-tie the knot, but just enough to prevent bleeding.
- Add a third (safety) ligature: As the last ligature is right on the edge, a third or safety ligature can be placed around the catheter and vessel, should there be any bleeding. Do not tie this off, as it could damage the pressure sensor.
Now the clamp can be slowly released.
- As there is some bleeding when the catheter is moved, the safety ligature will be tied off once the catheter has been advanced. This is a good example of what the safety ligature is for!
- Advancing the catheter into the ventricle, you will see the PV loops appear in LabChart (or your own software). [Example: video at 12.48]
- Once the catheter is in position, leave it for a minute or two to let it stabilize.

Tip: If the loops don’t settle, you can use your fingers to rotate the catheter to improve the loops.
- Once the baseline loops are satisfactory, it’s always a good idea to tie off the catheter using the ends of the obstructive ligature.
- Next, the second knot needs to be placed on the safety ligature.
Completion of procedure
Finally, clean up the wound and remove the hooks. For long term recordings, a piece of gauze moistened with saline can be placed on top to prevent fluid loss.
We hope you found these surgical instructions useful.
Please visit our comprehensive Ventricular Pressure Volume + PV Loops Application page for specialized information and useful resources relating to Pressure Volume Loop applications and research.
If you have any questions, please contact your ADInstruments support representative who will be happy to provide specific information and assistance with your research requirements.
Return to Surgical Instruction Video Series Main Page »
Additional resources
- PV Tips and Tricks: Expert advice for measuring pressure-volume loops in mice »
- Cardiac PV Loop Data Analysis: Tips & Tricks (Webinar) »
- Optimizing your Blood Pressure and Pressure-Volume Recordings »
- Cardiovascular Assessment of Pressure-Volume and Blood Pressure »
- Introduction to PV loops: Understanding the PV loop and measures of cardiac function »
- Troubleshooting your Millar Catheter: Three common problems and how to fix them »
Find out more about Pressure Volume (PV) Loop
recording and analysis:
Everything you need for ventricular pressure volume loop recording and analysis, in one place »
LabChart software - PV Loop 2.5 for left and right ventricle studies
The PV Loop Analysis Software Module for LabChart is specifically designed for the analysis of in vivo ventricular pressure-volume data in small and large animals, or ex vivo, using working heart systems. Our PV loop module provides workflows for acquiring PV loop data from small and large animal models as well as tools for analyzing left and right ventricular PV data in real-time or post-acquisition. Contact us for more information.