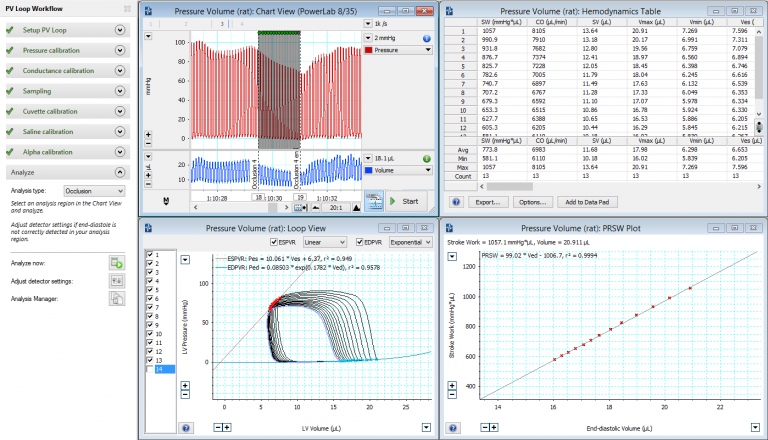
Left Ventricular Pressure-Volume via Carotid in Rat
Instructions on carotid artery catheterization using a Millar 2F catheter
for left ventricular pressure volume measurement in rats.
Surgery performed at the René Remie Surgical Skills Centre by Prof. René Remie. Animal handling and surgical procedures all strictly follow Dutch Legislation on Laboratory Animals and was approved by the IACUC.
Hardware:
- ADInstruments PowerLab 16/35 for data acquisition
- Animal BioAmp for ECG measurement
- Millar MPVS Ultra for Pressure and Volume measurement
Catheter:
- The Millar SPR-838 PV catheter is connected to the MPVS Ultra.
Software used to record and analyze the pressure signal:
- ADInstruments LabChart
- LabChart's PV Loop Module was used to record and analyze the pressure-volume signal.
Anesthetic:
The rat was anesthetized using injectable anesthetics and analgesics.
Procedure:
Make a simple catheter introducer
Before you begin the surgery, you can follow our step by step video instructions for making a simple catheter introducer from a 23 gauge syringe needle.
- With the bevel of the needle facing upwards, bend the tip almost 90degrees using a needle holder.
- You can also bend the needle a little to one side to prevent your fingers from being in the field of view when performing the catheterization.
- Attach the needle onto a syringe to create a simple catheter introducer.
Procedure begins: Incision
- Holding the tissue in the rostral direction, first make a small incision. Then using the tissue holder and scissors, make an incision along the neck.
- Open up between the two submandibular glands using a combination of sharp and blunt dissection.
- Moving between the two glands, and spreading the connective tissue, you will end up on the sternohyoid muscle, which can be clearly seen in the video at 02.56.
Find the carotid artery
As per the instructions on the video, three muscles will become visible:
- The sternohyoid muscle, going from the sternum to the hyoid bone.
- The sternomastoid muscle, going from the sternum to the mastoid.
- The digastric muscle, a muscle found between the previous two muscles.
These 3 muscles - sternohyoid, sternomastoid, and digastric - form a triangle.
Inside this triangle, you will find the carotid artery.

Tip: Approach the carotid through the sternohyoid muscle (as shown in the video). This method will provide enough artery length for a PV catheter, and will also give you more access to the carotid artery, which will be useful in the catheterization.
Make sure you create an opening length as long as possible, which can be accomplished using some micro scissors. This may cause some bleeding, but can be cleaned up using a cotton swab.
Separate the carotid artery
Once you have identified the carotid artery, you can hold back the tissue using simple hooks. With the tissue out of the way, you can clearly see the carotid artery.
- The first hook is placed as shown in the video at 05.30.
- The second hook is placed to move the muscle out of the way.
- The third hook is placed on the other side, retracting the other side of the sternohyoid muscle, and revealing the carotid artery below.
Now using some micro forceps and micro scissors, you will clear the tissue away around the artery.
- At the rostral end, you will find the bifurcation of the internal and external carotid artery.
- There is now more than a centimeter of exposed artery.
Ligatures
First, place the obstructive ligature as far rostrally as possible.
- Make sure that the suture does not include the vagus nerve, as tying it off with the artery will cause problems with the heart rate.
- The ligature will be placed just before the bifurcation.
- Tie off the knot.
- Using artery forceps on the ends of the obstructive ligature, hold the artery under tension.

Tip: Always keep the artery moist using some warm saline.
Place two more ligatures.
- One ligature will be used to tie off the artery around the catheter.
- The second ligature will be a safety ligature in case there is bleeding from the first ligature. Also, it can be used later on, to fixate the catheter once the catheter is in position.
Place the Beemer clamp.

Tip: To facilitate this you can lift the vessel by pulling on the second ligature. This will lift the artery and allow you to place the clamp as far caudally as possible. This will maximize the available length of the artery for catheterization.
Insert the catheter
- To insert the catheter, puncture a hole using the catheter introducer, as close as possible to the obstructive ligature.
- Place the catheter under the needle and advance it into the artery as far as it will go. This will allow you to tie off the ligature behind the last electrode.
- Don’t over-tie the knot, but just enough to prevent bleeding.
- With one hand holding the catheter, use your other hand to slowly remove the clamp, checking for any leaks.
Should it leak, just let go of the clamp and determine where the leak is. Otherwise, advance the catheter towards the heart using the tubing inducing forceps, or forceps with silicon tubing covered ends. - With the catheter in the artery, you can see the signal in LabChart (or your own software) where the dicrotic notch is clearly visible:
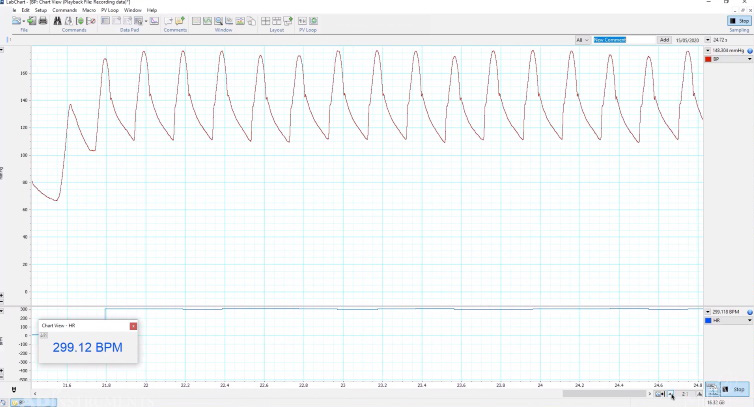
- As you advance the catheter it will eventually reach the heart. When it reaches the aortic valve, the catheter and vessel will move quite vigorously.
- If the catheter doesn’t enter the ventricle easily, it sometimes helps to move the animal slightly to the right, so that the catheter lines up better with the ventricle.
- Once the catheter moves past the aortic valve, you will see the resulting PV loops in Labchart (or your analysis software).

Tips:
- When doing a long term recording, it’s a good idea to tie off the extra ligature in case the second ligature has moved, or the vessel has been damaged due to the movement of the catheter.
- Use the obstruction ligature ends to tie off the catheter with another knot, so as to hold the catheter securely in place. In doing so, the catheter is held in 3 places and this will prevent it from moving.
Completion of procedure
To finish off the procedure, release the hooks and place a piece of gauze that has been moistened with sterile saline on top. This way you can record for some time, depending on the anesthetic used.
We hope you found these surgical instructions useful.
Please visit our comprehensive Ventricular Pressure Volume + PV Loops Application page for specialized information and useful resources relating to Pressure Volume Loop applications and research.
If you have any questions, please contact your ADInstruments support representative who will be happy to provide specific information and assistance with your research requirements.
Return to Surgical Instruction Video Series Main Page »
Additional resources
- Cardiac PV Loop Data Analysis: Tips & Tricks (Webinar) »
- PV Tips and Tricks: Expert advice for measuring pressure-volume loops in mice »
- Optimizing your Blood Pressure and Pressure-Volume Recordings »
- Cardiovascular Assessment of Pressure-Volume and Blood Pressure »
- Introduction to PV loops: Understanding the PV loop and measures of cardiac function »
- Troubleshooting your Millar Catheter: Three common problems and how to fix them »
Find out more about Pressure Volume (PV) Loop
recording and analysis:
Everything you need for ventricular pressure volume loop recording and analysis, in one place »
LabChart software – PV Loop 2.5 for left and right ventricle studies
The PV Loop Analysis Software Module for LabChart is specifically designed for the analysis of in vivo ventricular pressure-volume data in small and large animals, or ex vivo, using working heart systems. Our PV loop module provides workflows for acquiring PV loop data from small and large animal models as well as tools for analyzing left and right ventricular PV data in real-time or post-acquisition. Contact us for more information.