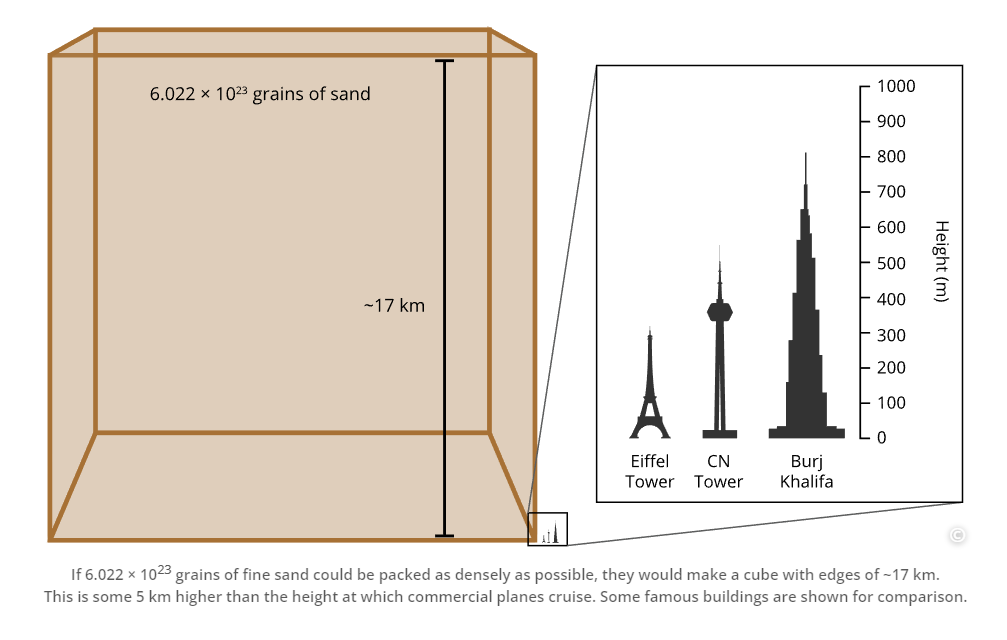
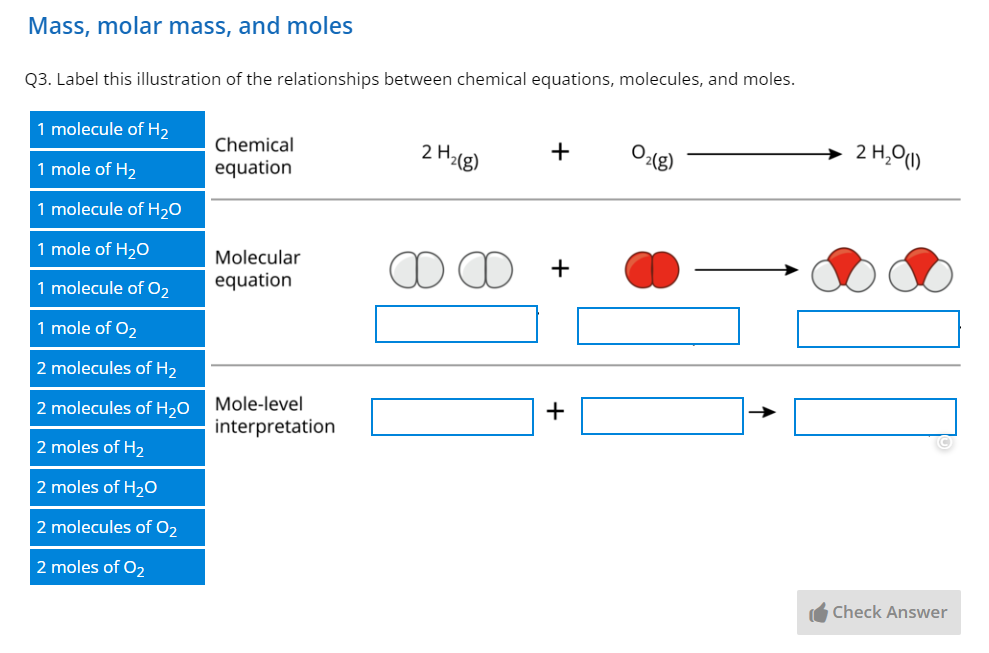
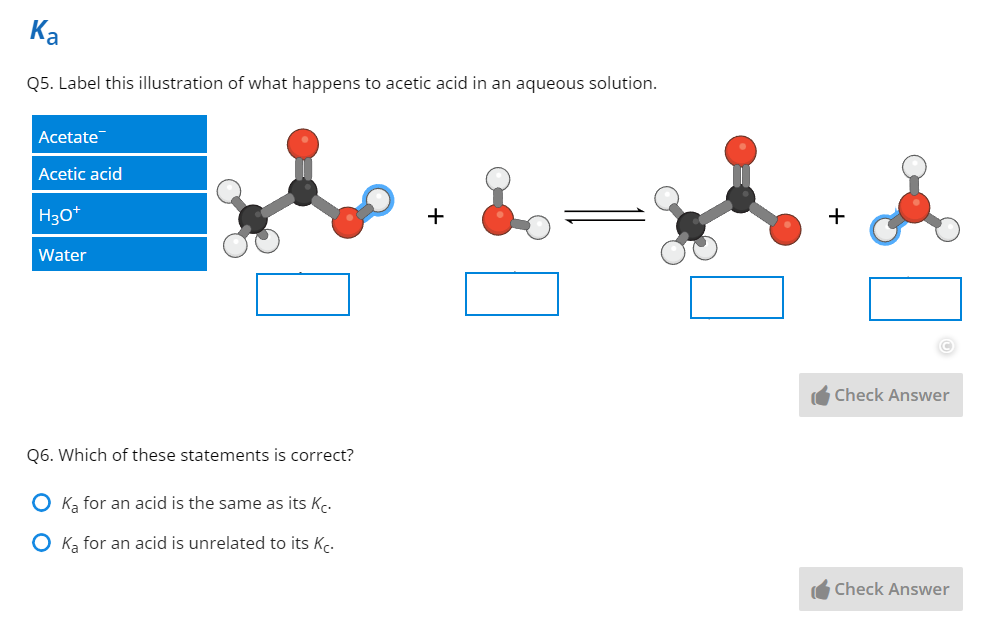
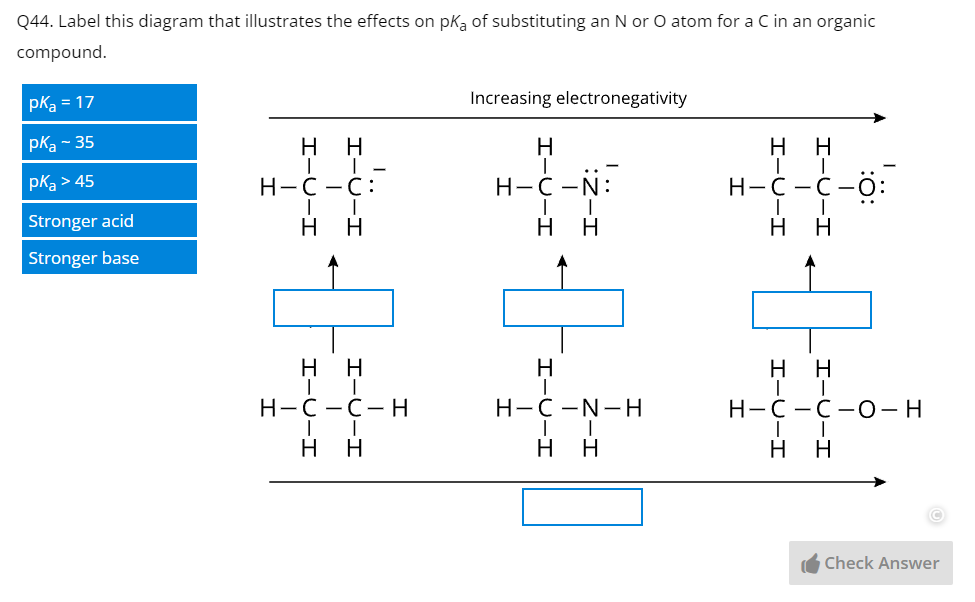
Through over 40 years of teaching experience, the late Professor Tony Macknight (co-founder of ADInstruments) developed a clear understanding of how students best learn, retain, and apply their knowledge. These key insights became the guiding principles of our new Understand Your Chemistry Collection - a comprehensive online-learning resource for first-year general chemistry courses.

"We know how students best learn - by a process of applying what they are learning to answer questions, and actively accumulating their knowledge and understanding."
Creating Understand Your Chemistry: an extensive collection of chemistry problems
After the successful launch of the Understand Your Physiology active-learning resource, Tony turned his focus toward chemistry: a subject often poorly understood and retained by students.
“Do your students enjoy chemistry? Do they like the textbook, and do they even use it? I believe many students get overloaded with information and confused about what they should know versus what information is for interest only,” Tony said.
“This is a structured learning resource with integrated calculations, interactive chemistry questions, and visual imagery. It’s designed to get students to understand the basic principles of first-year chemistry. “
Tony’s aim in developing Understand Your Chemistry was to produce an online, active-learning resource that taught chemistry at a level appropriate for a first-year general chemistry course. The resource needed to increase the relevance of chemistry to students’ lives and academic goals, and build a strong foundation for their future study. One way to build student understanding is to engage them in active learning and spaced repetition.
Below, Vicky from our instructional design team walks you through one of our lessons on weak acids and bases. If you’d like to interact with the lessons yourself in Lt, you can see inside the Weak Acids and Bases lab here.
Active learning builds students’ understanding of chemistry
Active learning is more effective for students’ overall performance and retention than lecturing alone. By conducting a meta-analysis of 225 studies, Freeman et al. found that the inclusion of active learning in undergraduate STEM classes increased performance by 0.47 standard deviations and reduced the rate of failure by 12%. Providing access to, and supporting, active learning is becoming increasingly important in modern education.
Understand Your Chemistry was created within Lt, an online platform designed for effective active learning. The broad range of question types available, the ease of constructing them, and the capacity to provide immediate and detailed student feedback meant that Tony could create an extensive and scaffolded set of lessons addressing a wide range of fundamental topics in chemistry. In building this comprehensive learning resource, Tony followed these active-learning principles:
- Build on what the student already knows.
- Before providing information, ask students to demonstrate their current knowledge.
- Then provide instant, quality feedback for all questions.
- Include repetition of important concepts with questions in different formats, so that students rehearse the information that they are learning.
- Provide contextual material so that students will be able to recall what they are learning when they need to use the knowledge.
- Allow students to learn at their own pace in their own time, wherever and whenever they can connect to the internet.
- Encourage students to study together in small groups rather than individually. Learning from each other is a powerful way to learn, understand, and retain knowledge.
Related: Find out more about Lt - our online-learning platform for life-science education »
Introduction to chemistry: a scaffolded journey through key general chemistry concepts
Understand Your Chemistry addresses core concepts in introductory physical and inorganic chemistry, suitable for students in a first-year general chemistry course. It has 40+ lessons, 1400+ questions, and 600+ illustrations and diagrams with which students actively interact, and there is no hardware required. We’ve designed the collection to be the ultimate flexible support to your course, saving you time.
The content is organized into nine modules:
Introduction | Covers the fundamental concepts that recur throughout the study of chemistry, including calculations, units of measurement, and naming conventions. |
Part A – Atomic Structure | Covers atomic models, electron configurations, molecular shape, bonding, and fundamental quantum mechanics. |
Part B – Chemical Equilibria | Covers molarity and mass, stoichiometry, and equilibrium in response to change. |
Part C – States of Matter | Covers intermolecular forces, aqueous solutions, solubility, and the laws governing gases, liquids, and solids. |
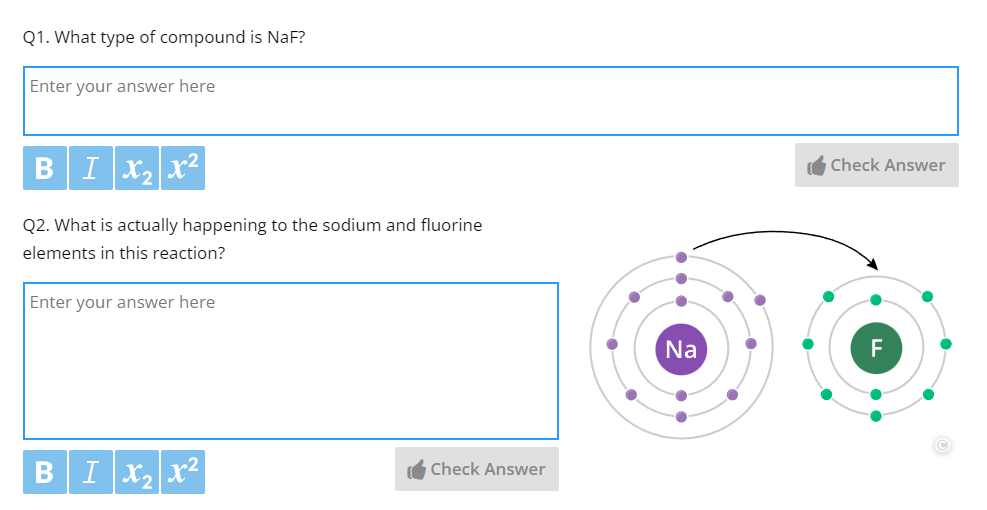
Part D – Acids and Bases | Covers acids and bases, weak acids and bases, buffer solutions, and acid-base titrations. |
Part E – Thermodynamics | Covers forms of energy, enthalpy, entropy, Gibbs energy, phase transitions, and the relationship between thermodynamics and life. |
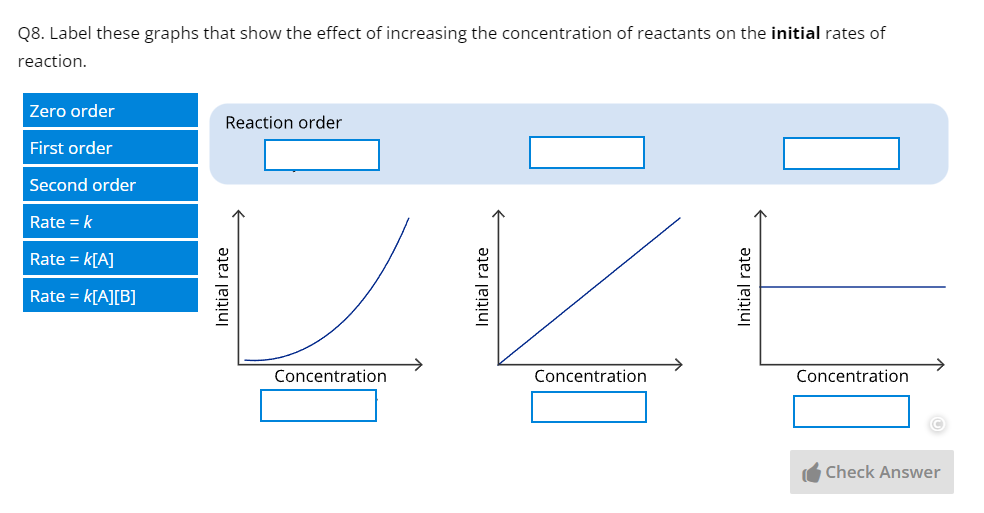
Part F – Kinetics | Covers the factors affecting rate, rate law, temperature and rate, reaction mechanisms, catalysts, and enzymes. |
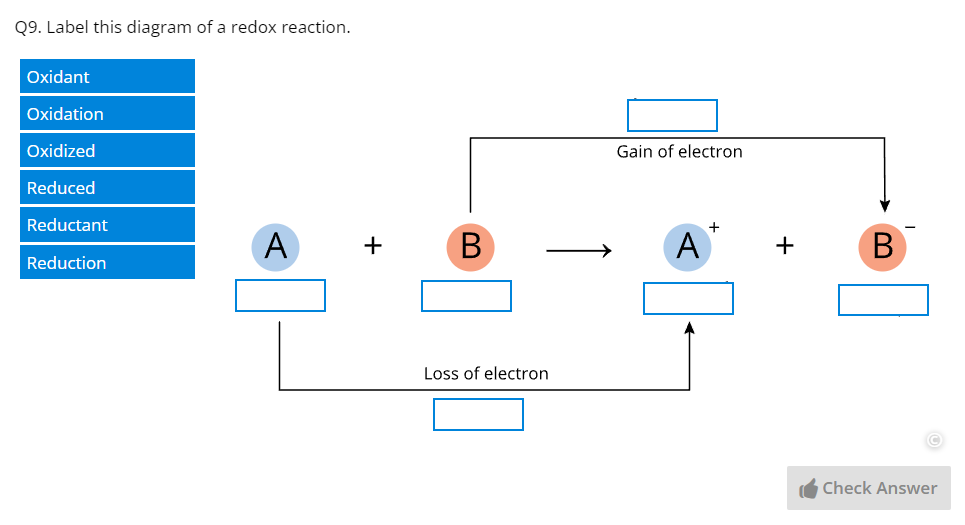
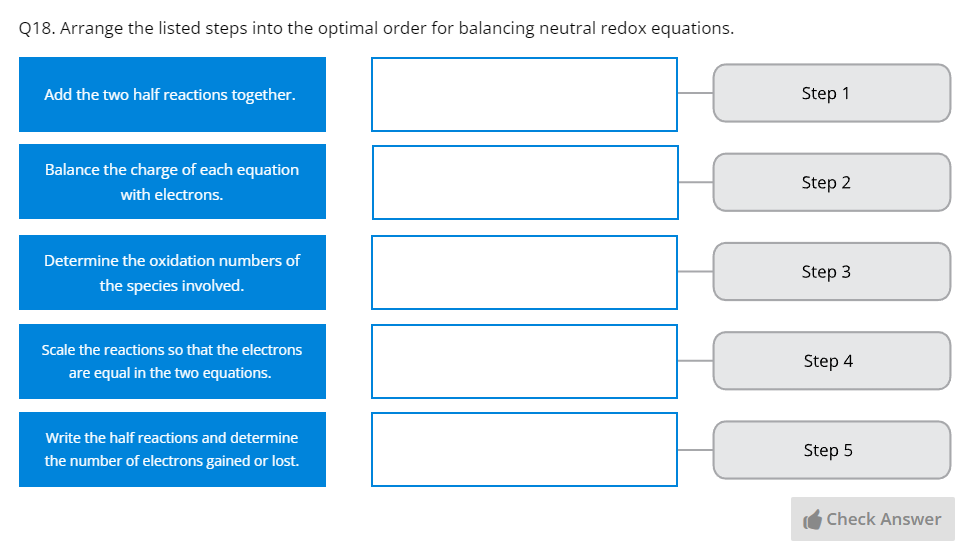
Part G – Redox Reactions | Covers the principles of redox reactions, electrochemical cells, and redox reactions in nonstandard conditions. |
Part H – Transition Elements | Covers the basic properties of transition metals and transition metal complexes. |
To ensure that students consolidate their understanding and adequately interact with key concepts, each module is followed by two chemistry review lessons to be completed one week and one month after completing the module. The overall structure of the resource means that dense topics are divided into manageable lessons to guide students through key concepts.
Building mathematical skills: a must for budding chemists
While students must become familiar with a range of new chemistry concepts, they must also build on a solid foundation of mathematics skills. Chemistry and mathematics are inextricably intertwined. We know that some students arrive at higher education with poor mathematical skills, which will negatively affect their progress in chemistry.
To address this issue, Understand Your Chemistry contains embedded calculation practice to improve students’ problem-solving ability. Students are set up for success in the introductory module, where they learn about units of measurement in chemistry, scientific notation, significant figures, simple algebraic calculations, and logarithms. Math is initially scaffolded, before scaffolding is removed to build skill.
Chemistry for beginners, developed by professionals
Tony Macknight was a Professor of Physiology at the University of Otago and conducted postdoctoral research at Harvard Medical School. His extensive teaching experience informed the design of Understand Your Chemistry, which in turn has been reviewed by chemistry educators and our in-house team of instructional designers. We are excited for Understand Your Chemistry to be used in general chemistry courses around the world, and as always welcome comments and suggestions that will improve the resource!
"Understand Your Chemistry does a thorough and smooth job of guiding students through the components of many important concepts in chemistry." - Jack Randall, B.A. Chemistry and Mathematics, M. Ed., Director of College Outreach, Vernier® Science Education.
Keeping track of student progress
Students can struggle with chemistry given its abstract nature and reliance on calculations. Analytics are an important part of keeping track of students' learning. The powerful analytic tools included in Understand Your Chemistry enable you to:
- Track student progress within lessons and sections.
- Quickly and easily identify students who are less engaged or who are having difficulties in the course, and so provide additional support for their learning.

- Identify questions which students struggle to answer; this diagnostic power enables you to target teaching within your course to address misconceptions or particularly tricky concepts.
How to use Understand Your Chemistry in your course
Tony believed that there are some fundamental principles that will determine your success in using this learning resource to promote student understanding of general chemistry. It is essential that you teach your course, not ours.
Nothing will work unless:
- You believe in what you are doing.
- The students believe that you believe in what you are doing.
- The time, effort, and commitment the students put into their study is seen to be rewarded.
This last point relates especially to the ways you decide on grades in the course. The ways you choose to examine the students determine the ways that they study. You have over 1400 questions to choose from here, so use some of these, or variants of them, in your tests and exams. This will ensure that what your students are studying and what they are tested on are in alignment.
Some fundamentals that will help you and your students make the most of this resource:
Encourage students to complete the ‘Student Introduction’ lesson
By sharing how the resource has been designed and why it will assist them to learn and to understand, we aim to make students comfortable using what, for many of them, will be a new way of learning. The student introduction teaches students how to 'study smarter’ and emphasizes the importance of spaced retrieval practice.
Integrate Understand Your Chemistry into the workflow of the course
This resource can be used flexibly to support your lectures and laboratories, and as a reference point for students to continue learning outside of the classroom. Because Understand Your Chemistry is constantly available to students online, they will be able to take part in active learning and receive feedback on their work at any time - even off-campus. Some ideas for how you might use the interactive lessons in Understand Your Chemistry include:
- Use as the online component of a blended-learning course. Share Understand Your Chemistry with your students before the semester starts as a course introduction; as pre-lab or lecture preparation; post-lab or lecture revision; or as a supplement for in-person sessions, for example, as an in-class chemistry quiz.
- Lessons can form the basis of tutorials. Students can work through lessons under your supervision when faced with particularly difficult concepts.
- Assign lessons as revision units when it's exam season. Because Understand Your Chemistry can be accessed anywhere there’s an internet connection, students can take the collection with them on their phones and laptops, and feel reassured that they can revise for their exams anywhere, anytime.
Lt's flexible, cloud-based nature lets students learn and receive feedback anywhere.
- Enable group work. The Lt platform allows students to login as a group to work together. This enables peer discussion and collaboration, so that students can learn from each other while they work through the questions. This is a powerful way to learn, understand, and retain knowledge.
Remix the lessons to tailor them to your course
Every one of the 40+ lessons in the Understand Your Chemistry resource is fully-editable, so that you can jump right in and change content to suit your curriculum. You might want to create mini quizzes for spaced repetition throughout the semester.
Find out more: How to quickly and easily edit content in Lt »
This is an exciting day for ADInstruments. We believe that we are providing a novel, original, and effective learning resource that will support students in mastering chemistry and provide them with strong foundations for future success. We’re confident that Understand Your Chemistry, like Understand Your Physiology before it, will improve student learning and understanding.
Please do contact us if we can assist you in any way in your teaching. If you’d like to ask a question about Understand Your Chemistry, you can do so here. If you’d like to jump straight in, you can use
Understand Your Chemistry for free for 90-days.
References:
Freeman, Scott, et al. “Active Learning Increases Student Performance in Science, Engineering, and Mathematics.” Proceedings of the National Academy of Sciences, vol. 111, no. 23, Proceedings of the National Academy of Sciences, May 2014, pp. 8410–15. https://doi.org/10.1073/pnas.1319030111.