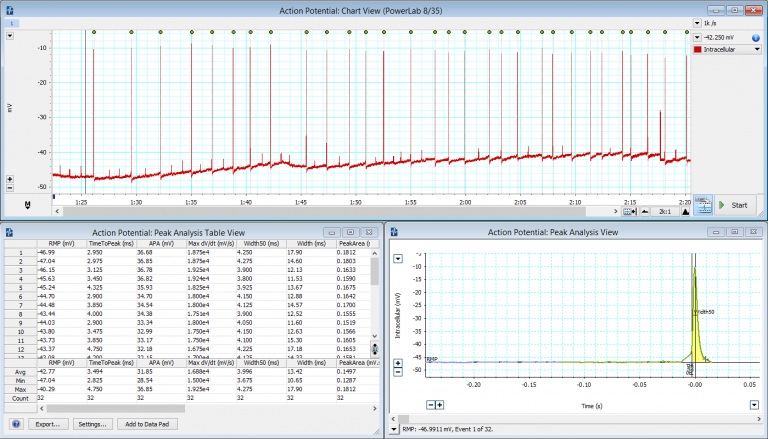
There are several variations of intracellular techniques, which can be grouped by the type of glass microelectrode used. The first group uses a microelectrode with a relatively small tip (fine or sharp), while the second uses a relatively large tip (blunt or patch). Each technique uses Ohm’s Law to focus on various aspects of the electrical activity.
Sharp Electrode
Membrane Potential/Voltage (Current Clamping): This technique measures the membrane potential by injecting a constant electrical current through the recording electrode, which is usually done to probe the passive or active properties of the cell membrane. The amplifier involved is designed to compensate for any resistive artifacts by the microelectrode. Current injection can also be used to introduce charged substances into the cell (iontophoresis), which can be used in cell marking techniques.
Membrane Current (Voltage Clamping): Classical voltage clamping involves the use of two electrodes (Two Electrode Voltage Clamping or TEVC), whereby one electrode records intracellular voltage and the other electrode injects current. The voltage-clamp amplifier forms a feedback circuit between the recorded voltage and the injected current. This then allows changing the cell membrane voltage to various held values, while recording the associated injected current required to realize the corresponding ‘clamped’ voltage. TEVC is most commonly used on large cells such as oocytes, and thus is sometimes referred to as oocyte clamping. Another voltage clamping technique is Single Electrode Voltage Clamping (SEVC), or sometimes known as switched clamp, uses a time-sharing technique that alternates between recording voltage and injecting current in a single intracellular electrode. It uses high frequency digital switching circuits, where the recording time is a short and fast enough that the cell remains ‘clamped’.
Patch Clamping
Unlike conventional sharp electrode techniques that involves impaling a cell, the patch microelectrode is placed next to a cell, and gentle suction is applied to draw a piece of the cell membrane (known as the 'patch') into the microelectrode tip, creating a high resistance (gigaohm) seal with the cell membrane. Variations on the suction can provide a variety of patching techniques including cell-attached, whole cell (similar to sharp electrode), outside-out patch and inside-out patch. Depending on the patching techniques, currents passing through individual ion channels, or through the whole cell can be recorded, or pharmacologically analysing the patch’s membrane properties.