Spinal cord injury is a deceptively complex condition. For outsiders, it appears to be just an issue of paralysis, a weakening or loss of movement from the site of the injury downward, but for those living with the injury, paralysis is often the least of their worries.
“Once your spinal cord is damaged your body loses the unconscious functions you take for granted,” Associate Professor Aaron Phillips says, “blood pressure stability, bladder and bowel control, sexual function; it all goes out the window.”
Aaron leads the Phillips Lab at the University of Calgary, focussing primarily on hemodynamic instability after spinal cord injury.
Hemodynamic instability is the inability of the body to maintain consistent blood flow and pressure. This instability leads to repeated hypotensive episodes; increasing the risk of stroke and heart disease, while seriously impacting recovery from spinal cord injury. Although pharmacological treatments are available, they increase the risk of complications and often have poor adherence. Aaron and his team wanted to find a solution that could directly target the injured system with high efficacy and negligible side effects.
They started with a rat model, tracking the descending pathways that regulate hemodynamics from the brain through to the spinal cord. Once the injury was introduced at T3, the neurological control of the cardiovascular system ended where the damage began. Using Kaha SNA and Pressure Telemeters they measured long-term concurrent activity from the sympathetic renal nerve and arterial blood pressure, revealing how the sympathetic and cardiovascular systems were interacting.
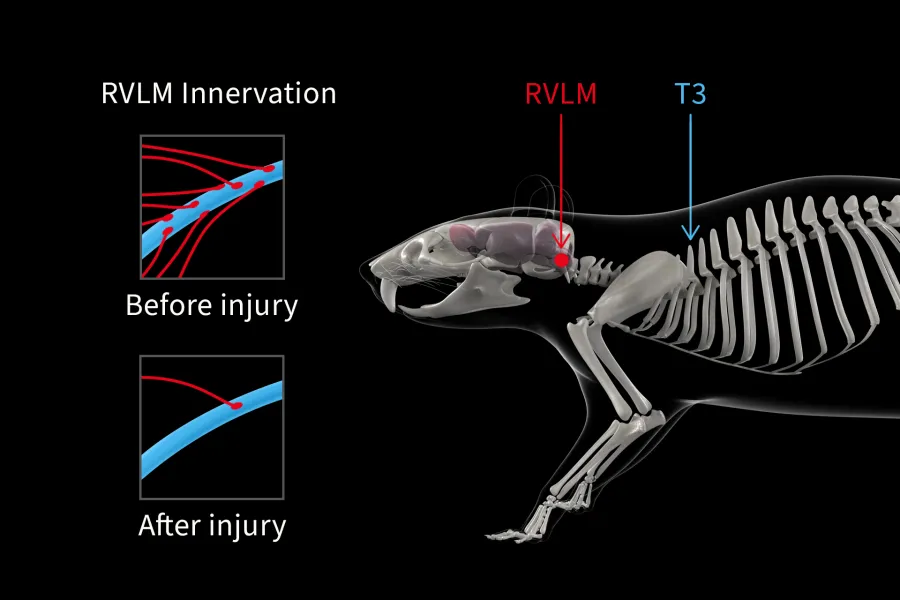
“It allowed us to understand how those systems responded to perturbations over time, in the normal daily living of freely moving animals.” Aaron says, “That insight was crucial in characterizing the model.”
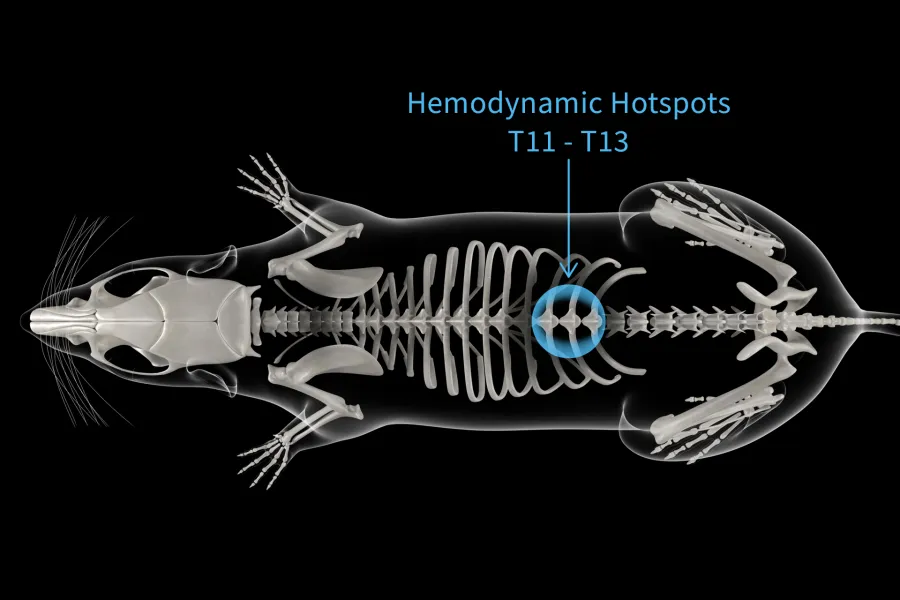
With a solid foundation to work from, the team moved on to interventions. Each segment of the spine was stimulated, and the hemodynamic effect was measured in order to find the right area for an implant. Everything pointed them toward T12. Mimicking natural signals sent from the brain, they repeated the experiment; it worked even better. No matter the challenge they threw at it, their neuroprosthetic baroreflex was consistent; reliably stabilizing previously unstable hemodynamics.
That reliability hasn’t faltered, not after being adapted for use in non-human primates, or being adapted again for use in humans. In fact, with the long-term implantation of the neuroprosthetic, a human patient was able to end medical treatments for hemodynamic instability, and completely resolve their orthostatic hypotension. It’s life-changing.
“In research, you’re often gathering huge amounts of data, and you don’t get to see whether or not your experiments have worked until it’s all been analyzed,” Aaron says, “But the big finding here is that this just works. It elevates blood pressure every time, it works across species, it works in the acute and chronic setting; it just works.”
More on the Neuroprosthetic Baroreflex
Deep Dive: The science behind the Neuroprosthetic Baroreflex
Squair, Jordan W., et al. “Neuroprosthetic Baroreflex Controls Haemodynamics After Spinal Cord Injury.” Nature, vol. 590, no. 7845, Jan. 2021, pp. 308–14. https://doi.org/10.1038/s41586-020-03180-w
Soriano, Jan Elaine, et al. “Longitudinal Interrogation of Sympathetic Neural Circuits and Hemodynamics in Preclinical Models.” Nature Protocols, vol. 18, no. 2, Nov. 2022, pp. 340–73. https://doi.org/10.1038/s41596-022-00764-w

