The isolated Langendorff heart has been a cornerstone of cardiovascular research for more than a century. Its long-standing scientific relevance is partly due to the fact that it allows experimental conditions to be studied beyond confounding hormonal and neurological influence. In addition, the isolated perfused heart serves as a bridge between in vivo and in vitro research, with many applications in heart physiology and pharmacology.

Research Fellow, Dr. Melanie White from the Univesity of Sydney, regularly applies the Langendorff technique within her research; studying how the heart behaves when faced with major physiological challenges such as myocardial ischemia.
We asked Melanie her to share her expert knowledge when it comes to using the Langendorff technique. Here Melanie will discuss best practices and key considerations when excising, cannulating and perfusing the heart, as well as handy tips for defining exclusion criteria and monitoring functional outputs.
(Warning - video contains dissection footage from a rat model)
Related: 12 Expert Tips for Langendorff Technique >>
Important considerations
Donor animal
An important consideration when designing your Langendorf experiment is what species you will use as the donor. The majority of research has been done using small rodent models (mainly mice and rats) due to their economic viability.
Larger rodents and small mammals such as guineas pigs and rabbits are also a popular option, as their vasculature is significantly larger than a mouse or rat, making the cannulation step a lot easier. However, bear in mind that larger animals are generally more expensive to house and have different handling requirements.
The sex and the age of the animal can also affect your experiment depending on the question you are asking. For example, females can have smaller infarct zones, which may be an issue if you are using an ischemia-reperfusion model.
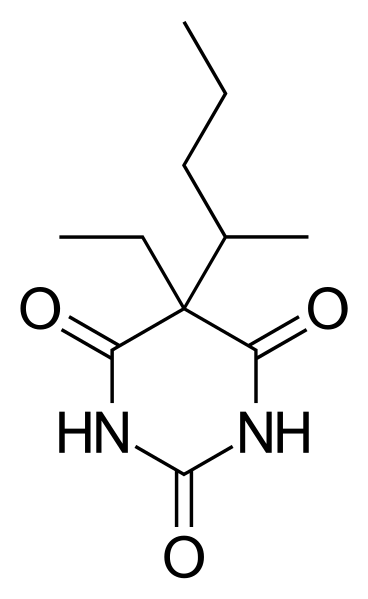
Choice of anesthesia
Choosing an appropriate anesthetic is important. The two main groups of anesthesias used are inhalants and injectables, the latter administered by an intraperitoneal or intravenous mode of delivery.
Normally we use barbiturates (e.g. pentobarbital), which is rather common in the field. However, it's important to note that there’s a narrow therapeutic range for the barbituates in order to apply a deep enough sedation but avoid cardiorespiratory suppression. We also apply heparin (an anticoagulant) to reduce clotting of the blood supply. This can either be delivered with the anesthesia during sedation or it can be applied separately.
Excision of the heart
Before performing any surgery on the animal, it's important to make sure that you have applied a deep enough anesthesia. You can do this by checking for an auditory response and a pain reflex on the footpad of the animal.
To remove the heart, you will need to perform a thoracotomy to expose the heart and the great vessels. During the excision procedure, it is important to dissect the aorta above the root, but below the aortic arch. This will provide you with a sufficient amount of the aorta to perfuse the aortic sinuses and in turn, the left and right coronary circulation.
Once excised, the heart is immediately submerged into a cold cardioplegia solution, to limit the effects of the hypoxic period which occurs during excision. We try to minimize our time from the opening of the diaphragm to the hanging of the heart to around about 90 seconds, but at a stretch, we'll go to 120 seconds.
Cannulation
Cannulation of the heart is by far the most challenging step of the procedure and is ultimately dependent on the size of the animal. In general, the larger the animal, the easier it is to cannulate the aorta. Once the heart is securely ligated on the cannula (but at least two or more sutures), you can gradually increase the flow rate from ~2mls/min in a rat model to 13 to 15 mls/min, allowing perfusion to commence. See here for information on Langendorff perfusate types.
At this point, you can trim away any additional tissue from the heart. This will help to avoid unnecessary force that would drag the heart down the aorta and create additional pressure.
Now you are in a position where you can insert a balloon into the left ventricle, allowing you to monitor the systolic and diastolic pressure developed in the heart. We normally set the balloon up with and afterload of 10 - 15 mmHg, as over time the heart will relax on the Langendorff and the diastolic pressure will decrease. Ideally, the heart will have established its bottom diastolic level within the baseline period.
Baseline period
Baseline periods traditionally run for fifteen to twenty minutes, allowing for the washout of any toxic metabolites that have been produced by the heart during the excision process. It also provides you with the opportunity to observe how the heart is functioning at an individual level and decide whether it can be included in the study beyond this point. As not all hearts will behave the same, having certain exclusion criteria in place (highlighted below) is incredibly important when performing isolated heart studies.
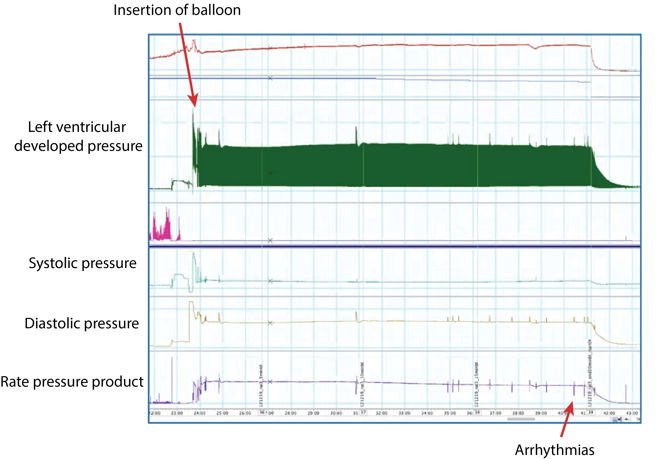
The picture above shows a zoomed-out view of a 20-minute baseline period (Recorded in LabChart). Upon insertion of the balloon, we start to record both our systolic and our diastolic pressure. We also have our left ventricular developed pressure (LVDP), which is automatically calculated in LabChart by minusing the end-diastolic pressure from the systolic pressure.
At the bottom, we have our Rate Pressure Product (RPP) which is a calculation based on our LVDP and the heart rate. You can see that there's only been a few little arrhythmias during this 20 minute period, therefore this particular heart would be viable to include in the study.
Minimum and maximum exclusion criteria
When assessing whether your heart should be included in your study, it’s important to have defined minimum and maximum exclusion criteria. For our minimum exclusion criteria, it would be defined by the time between excision and perfusion being greater than 120 seconds. Please note that this is the time from the incision into the diaphragm (during excision) - not the delivery of the anesthetic.
In our experiments, we also use exclusion criteria defined by the rate pressure product (RPP). For example, if we had a RPP less than 26,000, it would suggest that the heart is not performing adequately, even at the end of the baseline period.
Additionally, if the heart requires less than 10 mls per minute to have an adequate perfusion pressure, this would also be a reason for exclusion. Lastly, if the heart has numerous arrhythmias during the baseline period, you may want to consider excluding it from your statistical analysis.

Measuring functional outputs
What are some of the functional outputs you should be looking to measure?
- Perfusion pressure
- Perfusion temperature
- Flow Rate
- Heart Rate
- Diastolic and systolic pressure
- ECG/EKG for information on the development of the actional potential
- Calculate left ventricular developed pressure (LVDP)
- Calculate Rate Pressure Product (RPP)
You can record and analyze these cardiac parameters in real-time, using ADInstruments data acquisition hardware (PowerLab) and analysis software LabChart, as demonstrated in the diagram below. LabChart's additional acquisition and analysis features such as the Blood Pressure, ECG, HRV and Peak Analysis Add-Ons are particularly useful for Langendorff studies.
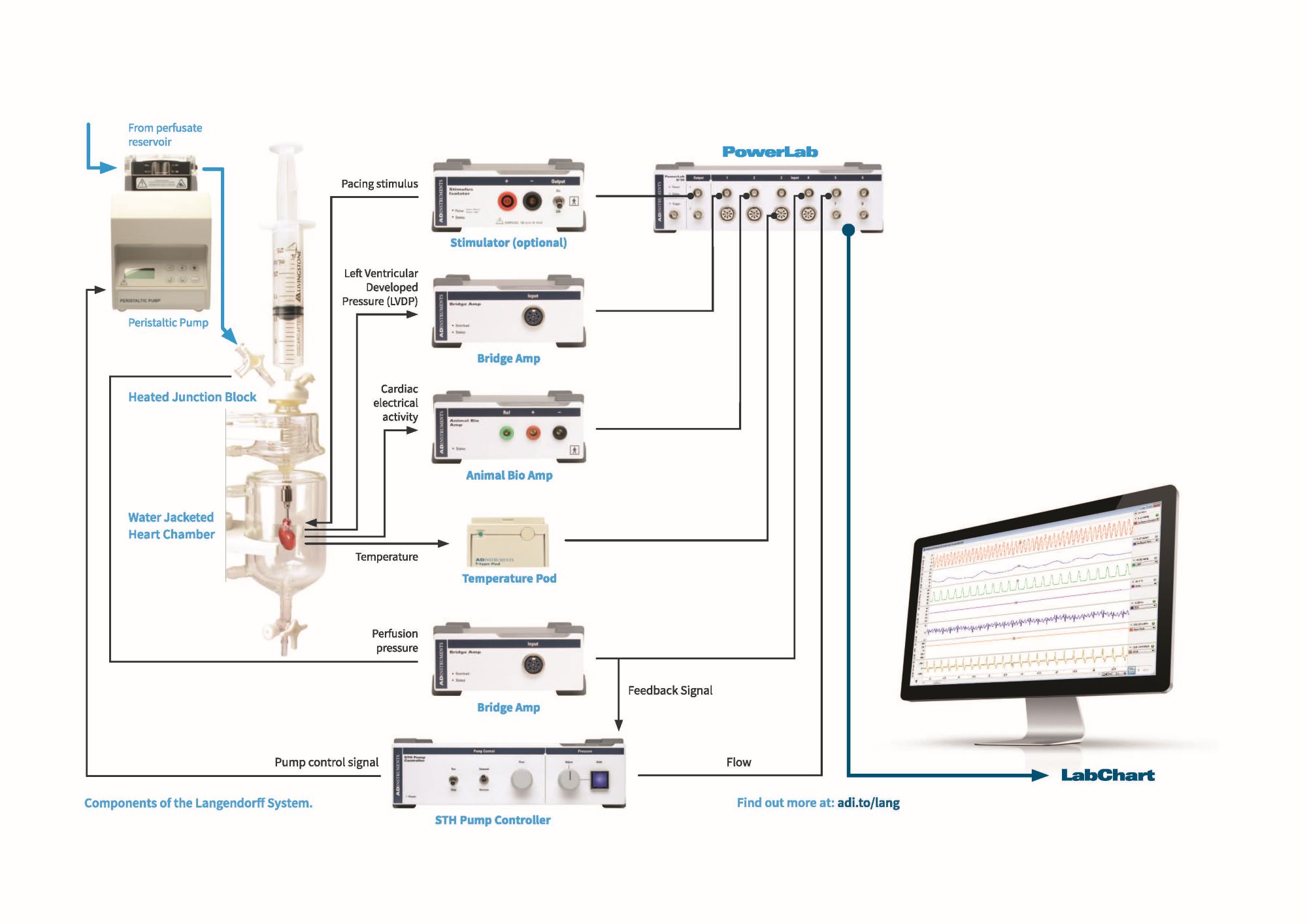
Example of a Langendorff heart set-up measuring functional outputs such as Left Ventricular Developed Pressure (Bridge Amp) and Cardiac electrical activity (Animal Bio Amp), as well as Perfusion pressure and temperature.
Click here to see the full webinar with Dr. Melanie white and learn about:
- Core principles of isolated Langendorff perfusion
- Key methodological considerations for excision, cannulation, and perfusion of the heart
- Tips for defining exclusion criteria, monitoring functional output, data analysis, and interpretation
- Applications of Langendorff perfusion, from myocardial ischemia to diabetic cardiomyopathy
Related:
Effects of constant flow vs. constant pressure perfusion on Langendorff isolated heart studies
Langendorff vs. Working Heart Perfusion – What’s the difference?
Choosing the correct perfusate for your Langendorff preparation
LabChart for Isolated Perfused Hearts (video)
Three benefits of isolated organ research
Key considerations when performing and isolated perfused liver experiment

